The Biological Basis of Myalgic Encephalomyelitis / Chronic Fatigue Syndrome
The disabling illness described as either Chronic Fatigue Syndrome (CFS) or Myalgic Encephalomyelitis (ME) results in a wide spectrum of symptoms, the most disabling often being a profound lack of energy, muscle pain, headache, and cognitive issues. The illness is also characterized by an increase in symptoms when in the upright position or following physical activity that would not be challenging to a healthy individual (post-exertional malaise). The factors that incite and perpetuate the illness are unknown, few treatment options exist, and full recovery is rare. We have previously investigated the possibility that a member of the murine retrovirus family could be involved. While that virus has been eliminated as a suspect, the existence of one or more inciting pathogens has not been ruled out. Well-documented outbreaks of ME/CFS implicate an infectious cause for at least some cases. Rapid onset following a viral illness has been reported, though many individuals recount a more gradual onset with no clear single provocation.
Project: Extracellular Vesicles in ME/CFS
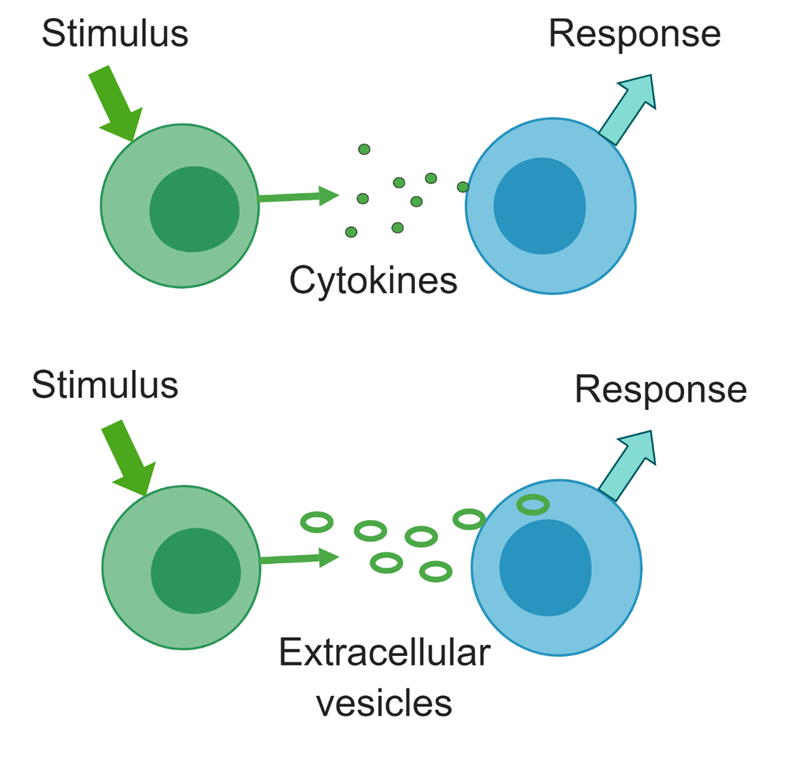 Cells signal each other through release and uptake of cytokines and extracellular vesicles (EVs). The three types of EVs are exosomes, produced through exocytosis, microvesicles, which bud from the cell membrane, and apoptotic bodies, which are products of dying cells. EVs carry both proteins such as cytokines and microRNAs, which can influence gene expression and activity of recipient cells. We are isolating EVs from ME/CFS patients and healthy controls and examining their protein, metabolite, and RNA cargo.
Cells signal each other through release and uptake of cytokines and extracellular vesicles (EVs). The three types of EVs are exosomes, produced through exocytosis, microvesicles, which bud from the cell membrane, and apoptotic bodies, which are products of dying cells. EVs carry both proteins such as cytokines and microRNAs, which can influence gene expression and activity of recipient cells. We are isolating EVs from ME/CFS patients and healthy controls and examining their protein, metabolite, and RNA cargo.
This project is funded through our NIH ME/CFS Center and more information can be found on the Center website:
https://neuroimmune.cornell.edu/research/vesicles-and-signaling/
Project: Post-Exertional Malaise in ME/CFS
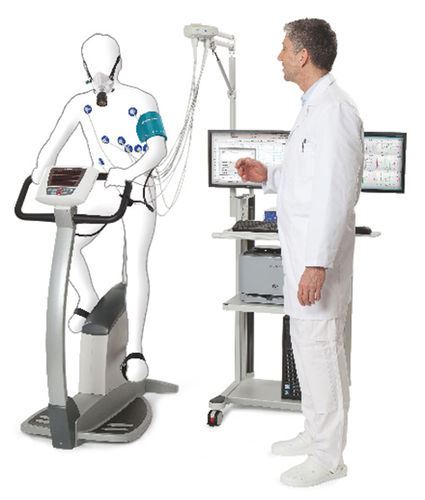
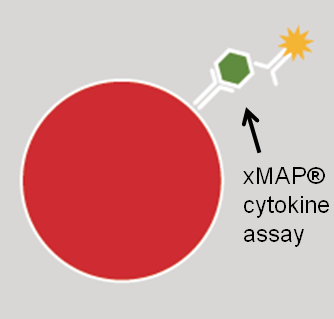 In collaboration with Dr. Betsy Keller in the School of Health Sciences and Human Performance at Ithaca College, we are examining the physiological and molecular basis of the exercise intolerance characteristics of CFS/ME patients. We are examining the effect of a maximal exercise stress test on gene expression, metabolism, inflammatory markers, and cytokine levels in ME/CFS patients and healthy controls.
In collaboration with Dr. Betsy Keller in the School of Health Sciences and Human Performance at Ithaca College, we are examining the physiological and molecular basis of the exercise intolerance characteristics of CFS/ME patients. We are examining the effect of a maximal exercise stress test on gene expression, metabolism, inflammatory markers, and cytokine levels in ME/CFS patients and healthy controls.
Project: Immune Metabolism
Immune cells adjust their metabolism when they transition from quiescence to activation due to highly regulated signals indicating a threat. If metabolic programs at rest and after activation are abnormal, immune cells will not be able to respond adequately. Dysfunctional response can prevent proper response to a pathogen and contribute to inflammation or autoimmunity. We are currently investigating fatty acid metabolism in immune cells from ME/CFS patients vs. controls, using both the Agilent Seahorse flux analyzer and flow cytometry.
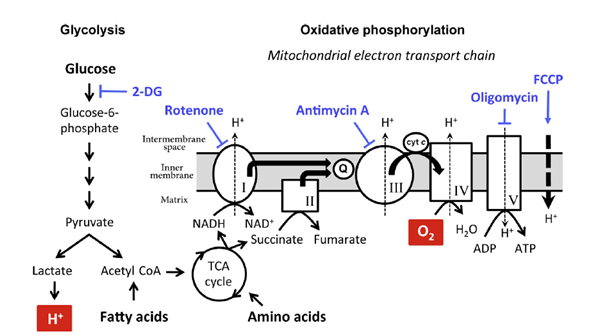
Previously, we examined glycolysis and oxidative phosphorylation as well as mitochondrial membrane potential in two types of T cells. This work demonstrated abnormalities in both types of T cells. We are carrying out additional studies of various types of T cells, B cells, and natural killer cells.
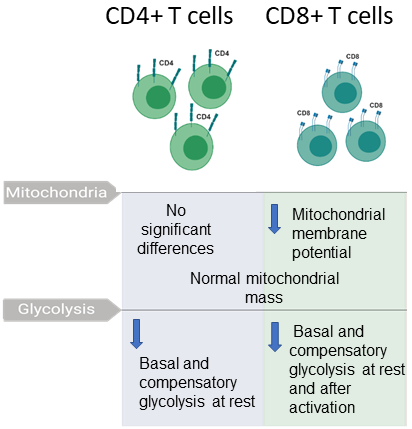
A summary of our published findings on immunometabolism of T cells from ME/CFS patients and controls. Samples were provided by Simmaron Research, Incline Village, NV.
Project: Plasma Proteomics of ME/CFS
Plasma contains a multitude of signaling proteins, especially ones involved in communication between immune cells or between tissues and immune cells. Plasma proteins may also provide insights into the function and stresses affecting tissue and organs. We have carried out a pilot study with 20 female ME/CFS patients and 20 controls using Somascan technology from Somalogic, which allowed us to study the relative abundances of 4790 unique proteins in plasma. Our published work describes the detection of abnormalities in the ephrin-eph pathway in ME/CFS. This pathway is involved in many biological processes such as axon guidance, angiogenesis, and epithelial cell migration. Furthermore, we detected dysfunction of immune signaling. The study indicated that combinations of plasma proteins may be able to serve as biomarkers of the disease.
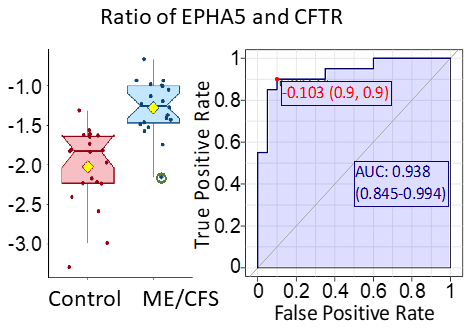
The ratio of the protein EPHA5 and CFTR gives a high level of discrimination between ME/CFS patients and controls, according to ROC analysis. Both of the proteins are involved in regulation of neurotransmitter release in response to varying blood glucose concentrations.
We are now engaged in further analysis of the proteome of plasma in ME/CFS, using a larger set of samples. We are again utilizing the Somascan technology as well as other methods.
Completed Project: Mitochondrial Genomes and ME/CFS
Dysfunction of mitochondria, the energy-generating organelle in human cells, is one hypothesis concerning the severe fatigue experienced by ME/CFS patients. To investigate whether there are genetic differences in the mitochondrial DNA of patients and healthy controls, we have sequenced the mitochondrial genome of a cohort of subjects recruited by the Chronic Fatigue Initiative.
 A publication for this project was published in the Journal of Translational Medicine:
A publication for this project was published in the Journal of Translational Medicine:
Mitochondrial DNA variants correlate with symptoms in myalgic encephalomyelitis/chronic fatigue syndrome.
by Paul Billing-Ross, Arnaud Germain, Kaixiong Ye, Alon Keinan, Zhenglong Gu, and Maureen R. Hanson
J. Translational Medicine. 2016, 14:19
Click here for a simplified explanation of the JTM publication.
Completed Project: The Microbiome and ME/CFS
 Many individuals with ME/CFS describe digestive disturbances. These symptoms might result from altered composition of the gut microbiome, as is known in intestinal inflammatory diseases. We have characterized the gut bacterial and eukaryotic microbiome in a cohort of ME/CFS patients vs. healthy controls, using 16S rRNA sequencing and 18S rRNA sequencing, respectively. We have observed a greater bacterial diversity in the ME/CFS patients and have observed differences in the taxonomic composition of the microbiomes of individuals and of the two populations.
Many individuals with ME/CFS describe digestive disturbances. These symptoms might result from altered composition of the gut microbiome, as is known in intestinal inflammatory diseases. We have characterized the gut bacterial and eukaryotic microbiome in a cohort of ME/CFS patients vs. healthy controls, using 16S rRNA sequencing and 18S rRNA sequencing, respectively. We have observed a greater bacterial diversity in the ME/CFS patients and have observed differences in the taxonomic composition of the microbiomes of individuals and of the two populations.
Results of the study are available here:



